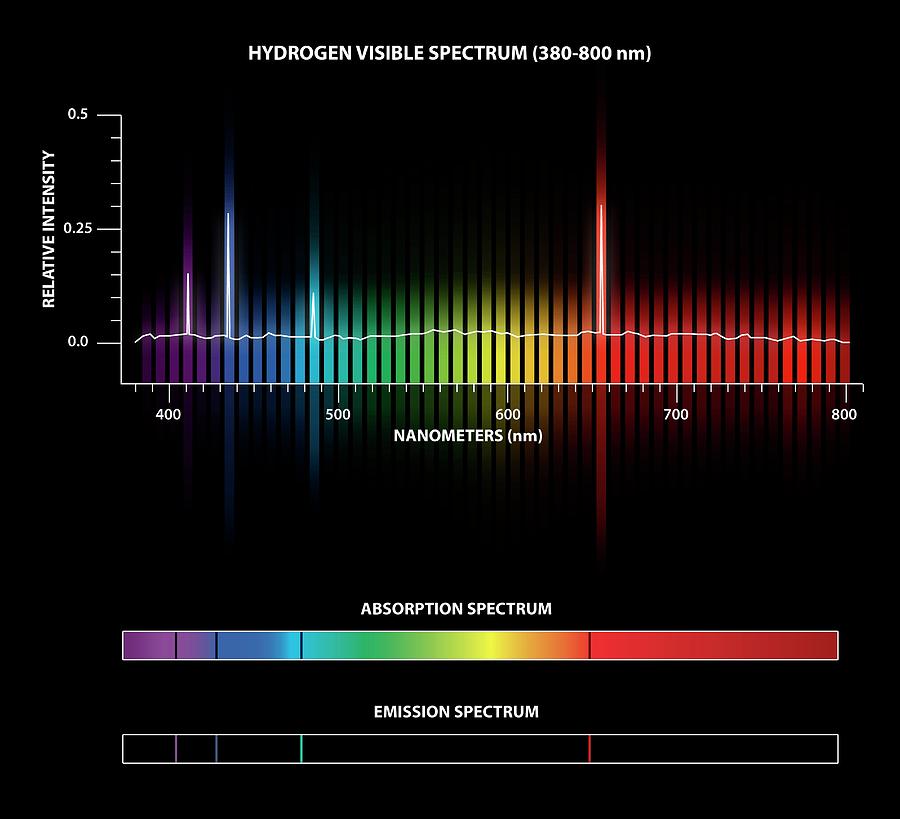
Emission Spectra Formula . the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. Let's calculate the energy of a single photon. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom.
from fineartamerica.com
Let's calculate the energy of a single photon. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom.
Hydrogen Emission And Absorption Spectra Photograph by Carlos Clarivan
Emission Spectra Formula Let's calculate the energy of a single photon. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. Let's calculate the energy of a single photon. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Formula the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within. Emission Spectra Formula.
From www.pinterest.com
emission spectra and energy levels Chemistry education, Physics Emission Spectra Formula the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Let's calculate the energy of a single photon. this spectrum of radiation emitted by electrons in the excited atoms. Emission Spectra Formula.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Formula the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. Let's calculate the energy of a single photon. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. the emission spectrum (or line spectrum) of a chemical element is the unique pattern. Emission Spectra Formula.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectra Formula this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. Web. Emission Spectra Formula.
From slidetodoc.com
ATOMIC SPECTRA Objectives 1 Determine the emission spectrum Emission Spectra Formula more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Emission Spectra Formula.
From www.researchgate.net
Emission spectra of the P1 solution (1 mg/mL) at different excitation Emission Spectra Formula the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. Let's. Emission Spectra Formula.
From chemwiki.ucdavis.edu
The Bohr Atom Chemwiki Emission Spectra Formula Let's calculate the energy of a single photon. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. any given element therefore has both a characteristic emission spectrum. Emission Spectra Formula.
From www.numerade.com
SOLVED Use the anthracene absorption and emission spectrum to Emission Spectra Formula any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. this. Emission Spectra Formula.
From www.researchgate.net
(a) Normalized emission, (b) actual emission, and (c) PLE spectra of Emission Spectra Formula Let's calculate the energy of a single photon. this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the rydberg formula is a mathematical expression used to. Emission Spectra Formula.
From www.researchgate.net
The formula (A) and the excitation and emission spectra of FMN in PBS Emission Spectra Formula this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. Let's calculate the energy of a single photon. more important, rydberg’s equation also predicted the wavelengths of other series of. Emission Spectra Formula.
From pressbooks.online.ucf.edu
5.3 Spectroscopy in Astronomy Astronomy Emission Spectra Formula Let's calculate the energy of a single photon. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. any given element therefore has both a characteristic emission spectrum and a characteristic. Emission Spectra Formula.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure of Atom Emission Spectra Formula the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. this spectrum of radiation. Emission Spectra Formula.
From edurev.in
Using the Rydberg formula,calculate the wavelengths of first four Emission Spectra Formula this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many.. Emission Spectra Formula.
From fineartamerica.com
Hydrogen Emission And Absorption Spectra Photograph by Carlos Clarivan Emission Spectra Formula the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. the emission spectrum (or line. Emission Spectra Formula.
From www.researchgate.net
The emission spectra of samples No. 3 (1) and 4 (2) excited by REB on Emission Spectra Formula Let's calculate the energy of a single photon. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the emission spectrum (or line spectrum) of a chemical element is the. Emission Spectra Formula.
From franklinhu.com
It can be extended to calculate the spectra of Hydrogen Emission Spectra Formula this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Let's calculate the energy of a single photon. more important, rydberg’s equation also predicted the. Emission Spectra Formula.
From www.pinterest.com
Absorption and emission lines Astrophysics, Space and astronomy Emission Spectra Formula this page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. Let's calculate. Emission Spectra Formula.
From www.youtube.com
The emission spectrum of the hydrogen atom and the Rydberg equation Emission Spectra Formula more important, rydberg’s equation also predicted the wavelengths of other series of lines that would be observed in the. the rydberg formula is a mathematical expression used to predict the wavelengths of spectral lines of many. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is.. Emission Spectra Formula.